AFIRMA THYROID CYTOPATHOLOGY/MOLECULAR ANALYSIS

Afirma® is a combined cytology evaluation/molecular analysis offered by Veracyte® Inc. for evaluation of thyroid nodules.
The test is intended to help triage surgical intervention in patients with thyroid nodules utilizing molecular analysis of FNA specimens that have an “indeterminate” result on cytology.
“Indeterminate” results for cuyology FNA samples include the following:
The test is intended to help triage surgical intervention in patients with thyroid nodules utilizing molecular analysis of FNA specimens that have an “indeterminate” result on cytology.
“Indeterminate” results for cuyology FNA samples include the following:
- Follicular lesion of undetermined significance (FLUS) - Bethesda category III
- Atypia of undetermined significance (AUS) – Bethesda category III
- Suspicious for a hürthle cell/follicular neoplasm – Bethesda category IV
How it works
1) Veracyte® provides clinician with collection kit and training for obtaining the sample.
2) Clinician performs FNA and sample is sent to Veracyte®
3) An independent lab partnered with Veracyte® prepares cytology slides where they read by a cytopathologist.
4) The cytopathologist interprets the slides as benign, malignant or “indeterminate”.
5) Samples that are benign or malignant do not get further molecular analysis. The diagnosis is reported to the clinician without further testing.
6) Samples that are interpreted as “indeterminate” are sent for molecular analysis.

Molecular Assay Methodology
Gene expression classifier (GEC) which consists of a 25 gene microarray to rule out uncommon malignancies such as medullary carcinoma and a main 142 gene microarray assay which is used for determining whether a thyroid nodule will be reported as “benign” or “suspicious”. The microarrays are analyzed according to a multi-dimensional algorithm. The specific details of the genes examined and the algorithm are proprietary information.
Resulting
After molecular analysis of “indeterminate” samples the clinician will receive an overall interpretation which is reported as either “Benign” or “Suspicious”.
Patients with a “Benign” result can often be followed up with ultrasound surveillance and avoid surgery.
Patients with a “Suspicious” result are generally referred for surgical intervention.
Performance
Veracyte® reports over 94% negative predictive value for patients that have an indeterminate cytology result and a “benign” Afirma® result following molecular analysis. They also report that patients with an indeterminate cytology and a “benign”
Afirma® result have a risk of malignancy comparable to patients with a benign diagnosis via cytology alone. Therefore, Afirma® testing is useful for identifying patients who may be able to avoid surgery that otherwise might be performed based upon indeterminate results via cytology alone.
References:
Link to Veracyte® website: Click here
Link to NEJM manuscript with clinical validation study results: Click here
PAPILLARY THYROID CARCINOMA
Compiled by the APMG Molecular Committee- NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
- BRAF
- SUGGESTED READING
NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy. It generally has a favorable prognosis, but PTC may result in mortality in up to 10% of patients. The BRAF V600E is the most common genetic alteration found in sporadic PTC.
The RAS-RAF-MEK-ERK signaling pathway (MAPK pathway) is a classical intracellular pathway that plays a crucial role in the homeostasis of normal cell turnover, cellular proliferation, differentiation, survival, and apoptosis. When activated aberrantly, this signaling pathway can induce tumorigenesis and has been associated with various malignancies.
MAPK Signaling Pathway
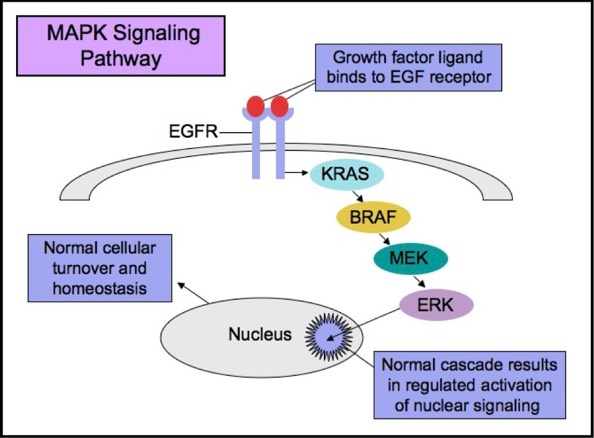
BRAF
BRAF is part of the RAF family of serine-threonine protein kinases, and a potent activator of the MAPK pathway. BRAF V600E mutations are detected in about 40% of PTC’s, including well-differentiated PTC’s, poorly differentiated thyroid carcinomas, and anaplastic thyroid carcinomas. Tumors that harbor areas of well-differentiated and poorly differentiated thyroid cancers typically show BRAF mutations in both components, indicating that BRAF mutation is likely involved in all stages of tumorigenesis, and potentially causes the cells to dedifferentiate.
BRAF mutations are usually not seen in benign thyroid entities or in other types of thyroid carcinomas such as medullary thyroid carcinoma and follicular thyroid carcinoma. The histologic variants of PTC most associated with BRAF mutation are conventional PTC with classic papillary architecture, tall cell variant, and papillary thyroid microcarcinoma.
Many studies have shown that BRAF V600E is a vital molecular prognostic marker for PTC. While PTC generally has a high cure rate and a good prognosis, studies show that patients with the BRAF mutation are more likely to have a poor prognosis, with advanced clinical stage, lymph node metastasis, extra-thyroidal extension, and mortality.
While there is no widely accepted method for detection of BRAF mutation in thyroid cancers, direct automatic sequencing is currently the gold standard. It has also been suggested that analysis of fine needle aspiration specimens for BRAF mutations in cases with equivocal cytology may be helpful in guiding individualized treatment.
Multiple ongoing trials, researching on tyrosine kinase inhibitors targeting the mutant BRAF protein, show promising success in their pre-clinical stages. With continued research, therapies with maximum anti-tumor activity and minimal toxicity may emerge in the future for treatment of aggressive PTC’s, including poorly differentiated thyroid carcinomas and anaplastic thyroid carcinomas.
SUGGESTED READING
Yaqiong L, Nakamura M, Kakudo K. Targeting of the BRAF gene in papillary thyroid carcinoma (Review). Oncology Reports 22: 671-681, 2009.
http://www.spandidos-publications.com/or/22/4/671

