LUNG CARCINOMA
Compiled by the APMG Molecular Committee- NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
- CLINICAL INDICATIONS FOR EGFR/KRAS/ALK/ROS1 TESTING
- PROPOSED ALGORITHM FOR EGFR/KRAS/ALK/ROS1 TESTING
- TEST COMMENTS
- TEST INTERPRETATION
- SUGGESTED READING
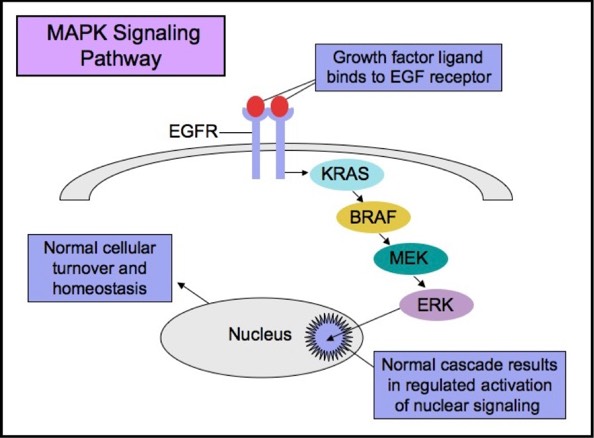
NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
The RAS-RAF-MEK-ERK signaling pathway (MAPK pathway) is a classical intracellular pathway that plays a crucial role in the homeostasis of normal cell turnover, cellular proliferation, differentiation, survival, and apoptosis. When activated aberrantly, this signaling pathway can induce tumorigenesis and has been associated with various malignancies.
MAPK Signaling Pathway
EGFR
The Epidermal Growth Factor Receptor (EGFR), also known as HER1 and ERBB1, is an important trans-membrane tyrosine kinase receptor involved in the initiation of the MAPK pathway. Activating mutations in EGFR can lead to aberrant cellular proliferation, inhibition of apoptosis, angiogenesis, increased cell survival, and gene transcription.
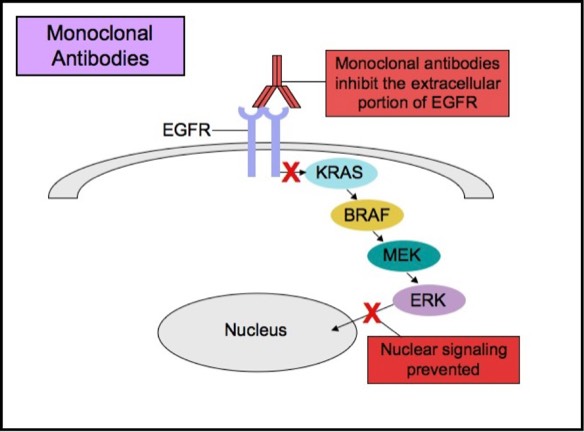
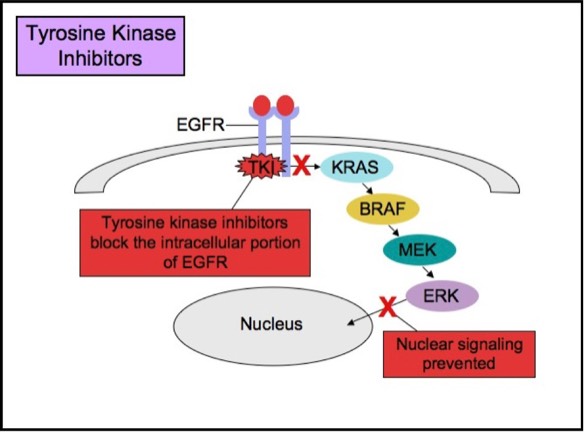
Some patients with tumors carrying EGFR mutations may respond to selective anti-EGFR therapies. EGFR has an extracellular ligand-binding region and a cytoplasmic tyrosine kinase-containing domain. Currently, there are two major types of anti-EGFR drug therapies. The first drug type (cetuximab, panitumumab) is a monoclonal antibody that inhibits EGFR by binding to an extracellular portion of the EGFR protein. The second type (gefitinib, erlotinib) is an intracellular tyrosine kinase inhibitor that inhibits EGFR by crossing the cell membrane and blocking the receptor’s active site.
Monoclonal Anti-EGFR Therapy
Tyrosine Kinase Inhibitor Therapy
Studies show that patients whose lung cancers exhibit EGFR mutations may benefit from anti-EGFR therapies. Tumor subtypes most likely to respond to anti-EGFR therapies include adenocarcinoma, non-mucinous bronchioloalveolar carcinoma (BAC), and adenosquamous carcinoma. EGFR mutations are rare in other histologic types of lung cancer (small cell carcinoma, squamous cell carcinoma, and large cell carcinoma). Thus, anti-EGFR therapies are most commonly employed for treatment of adenocarcinoma. The two most common EGFR mutations, comprising about 90% of all EGFR mutations in lung adenocarcinomas, are on exon 19 and 21 (L858R). Patients with these mutations are likely to respond to anti-EGFR therapies. There are also less common mutations (exon 20 insertion), including those that actually cause poor response to anti-EGFR therapies, but these mutations are relatively uncommon.
KRAS
KRAS is a small G-protein that is important for EGFR signaling. KRAS mutations activate downstream signaling cascade despite the upstream EGFR regulation. Anti-EGFR therapies are ineffective in tumors with such activating KRAS mutations, because the KRAS mutations trigger the EGFR-signaling cascade at a point downstream of the therapy’s target. Therefore, both monoclonal antibodies and tyrosine kinase inhibitors may fail to respond in the presence of KRAS mutations.KRAS mutations are seen in about 15-20% of non-small cell lung cancers and 30-50% of lung adenocarcinomas. EGFR and KRAS mutations are essentially mutually exclusive in lung adenocarcinomas. The presence of both EGFR and KRAS mutations in lung adenocarcinomas is rare, but if they do occur together, EGFR inhibitors are less likely to be effective. KRAS mutation is a poor prognostic indicator. The presence of KRAS mutation usually means failure to respond to anti-EGFR therapy. Anti-EGFR therapies are likely to be ineffective in patients with advanced or metastatic lung adenocarcinomas when a KRAS mutation is present.
In patients with lung adenocarcinoma, non-mucinous BAC, or adenosquamous carcinoma, EGFR mutation analysis by polymerase chain reaction (PCR) is recommended to detect those patients who may benefit from anti-EGFR therapy. Laboratories often offer EGFR mutation PCR test, with reflex to KRAS (if EGFR is negative), since the two mutations are mutually exclusive.
KRAS Mutations
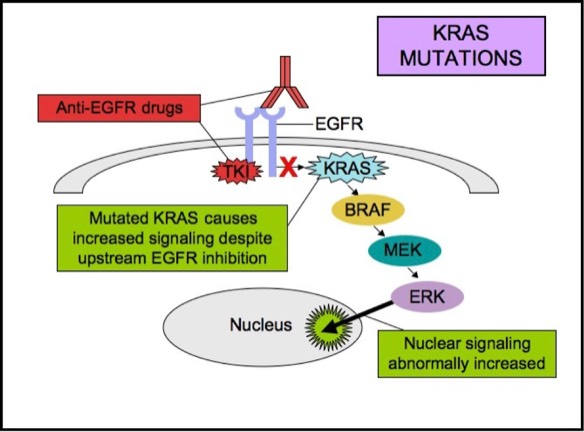
ALK
Non-small cell lung cancers have also been linked to rearrangements of the gene encoding anaplastic lymphoma kinase (ALK). The most common ALK rearrangement found in non-small cell lung cancers is the fusion between echinoderm microtubule-associated protein-like 4 (EML4) and ALK gene on chromosome 2p23. EML4-ALK non-small cell lung cancer is a unique subset of non-small cell lung cancer that is most commonly seen in adenocarcinomas of never or light smokers, whose tumors lack EGFR and KRAS mutations. Patients with ALK rearrangements tend to be younger than most patients with non-small cell lung cancer. These patients usually do not benefit from EGFR-specific therapy, but may benefit from specific ALK inhibitors. Crizotinib is an oral ALK tyrosine kinase inhibitor approved for advanced or metastatic ALK-positive non-small cell lung cancer (i.e patients who harbor the ALK gene rearrangement). FISH is the current standard method for determining the presence of the EML4-ALK gene translocation. EML4-ALK translocations, KRAS mutations, and EGFR mutations in lung cancer are almost always mutually exclusive.
ROS1
ROS1 is a receptor tyrosine kinase. ROS1 fusions are encountered in approximately 2% of non-small cell lung cancers and are identified as a potential driver mutation in non-small cell lung cancers. ROS1 rearrangement-positive lung cancer is a distinct subset of lung cancer with clinical characteristics similar to ALK-rearranged lung cancer. Similar to ALK rearrangements, ROS1 fusions are more commonly seen in light or never smokers. They are also associated with younger age and adenocarcinomas. ROS1 fusion-positive cancers are reportedly sensitive to tyrosine kinase inhibitors that inhibit ROS1. Crizotinib has shown early evidence of clinical response in ROS1 fusion-positive patients. FISH is the current standard method for detecting ROS1 rearrangement. ROS1 mutations are non-overlapping with other oncogenic mutations found in non-small cell lung cancer such as ALK, EGFR, and KRAS mutations.
CLINICAL INDICATIONS FOR EGFR/KRAS/ALK/ROS1 TESTING
Testing should be performed on:
- Patients with metastatic adenocarcinoma
- Patients with unresectable tumors
- Patients with recurrent tumors
- Patients who cannot tolerate conventional chemotherapy
- Patients with mixed lung cancers with any adenocarcinoma component in a lung resection specimen. For patients without any adenocarcinoma component by histology or IHC (i.e. pure SCC or small cell carcinoma) EGFR and ALK testing is not recommended
- Patients with limited specimens (i.e. core biopsy or cytology) where an adenocarcinoma component cannot be excluded and the clinical presentation is suspicious for adenocarcinoma (i.e. young age or absence of smoking history).
- Patients with multiple separate primary lung adenocarcinomas. Each separate primary may be tested, however, testing of different areas within the same primary adenocarcinoma is not recommended.
Acceptable specimens usually include:
PROPOSED ALGORITHM FOR EGFR/KRAS/ALK/ROS1 TESTING
Testing for EGFR, ALK, KRAS, and ROS1 mutations may all be ordered separately at the time of the resection. Alternatively, since all these mutations are essentially mutually exclusive, KRAS and ROS1 testing are often ordered as reflex, if EGFR or ALK1 is negative, respectively. I am recommending (as available) automatic reflex to KRAS and ROS1 when EGFR and ALK1 are negative. See algorithm below.
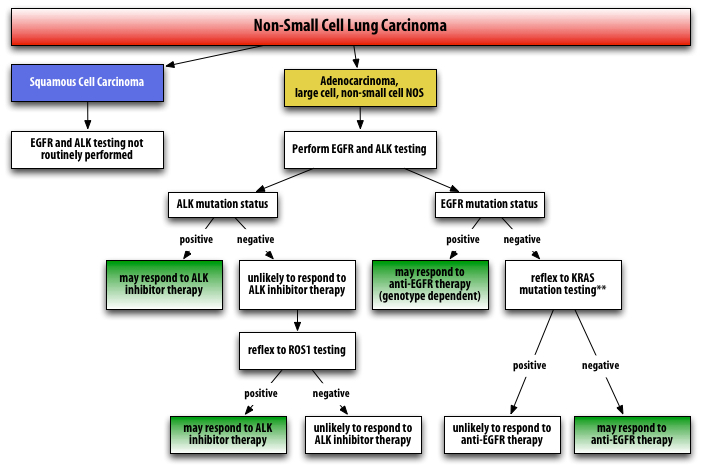
**Although it is counter-intuitive to pursue further testing in a patient who is EGFR- negative, determining the presence of KRAS mutation is still useful information because patients who are EGFR-mutation negative often are still candidates for anti-EGFR therapy as a second-line agent. Studies have shown that anti-EGFR therapy may be useful in patients with NSCLC as second-line, third-line, or as maintenance therapy, including those patients who are EGFR mutation-negative.
The above information is largely based upon the National Comprehensive Cancer Network (NCCN) recommendations and guidelines.
The above information is largely based upon the National Comprehensive Cancer Network (NCCN) recommendations and guidelines.
TEST COMMENTS
EGFR mutation analysis by PCR is available upon request. This test is intended to detect EGFR mutation-positive patients. Pulmonary non-small cell carcinomas, including adenocarcinomas, non-mucinous bronchioloalveolar carcinomas, and adenosquamous carcinomas with certain EGFR mutations may respond to anti-EGFR therapies. Alternatively, rare EGFR mutations predict failure to respond to anti-EGFR therapies.
EML4-ALK gene translocation and ROS1 fusion analysis by FISH are also available upon request. These tests are intended to detect patients who may respond to anti-ALK therapy.
KRAS mutation analysis by PCR is also available upon request. This test is useful in patients found to be negative for EGFR mutations. Patients with KRAS mutation may also show relative insensitivity to anti-EGFR therapies that target the RAS/RAF pathway.
TEST INTERPRETATION
PCR GENE MUTATION ANALYSIS INTERPRETATION:
EGFR Mutation Analysis:
Positive = Mutation Detected; specify genotype*
Negative = Mutation Not Detected
*When an EGFR mutation is detected, a specific genotype is reported. Although most EGFR mutations predict a favorable response to anti-EGFR therapies, rare mutations are associated with a lack of clinical response to anti-EGFR therapies.
KRAS Mutation Analysis:
Positive = Mutation Detected: Unlikely to respond to anti-EGFR therapy
Negative = Mutation Not Detected: May respond to anti-EGFR therapy
ALK Fusion Analysis:
Positive = Fusion Detected: May respond to anti-ALK therapy
Negative = Fusion Not Detected: Unlikely to respond to anti-ALK therapy
ROS1 Rearrangement Analysis:
Positive = Fusion Detected: May respond to anti-ALK therapy
Negative = Fusion Not Detected: Unlikely to respond to anti-ALK therapy
SUGGESTED READING
Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Modern Pathology 21: S16-S22, 2008.
http://www.nature.com/modpathol/journal/v21/n2s/full/3801018a.html
CAP/IASLC/AMP: Molecular Testing Guideline for the Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors. Archives of Pathology 2013, 137:828-860
CAP Lung Molecular Reporting Template
CAP Webinar on lung cancer testing can be viewed here:
CAP Resource Page on Molecular Testing in Lung Cancer
CAP Today article on lung cancer molecular testing.
CAP Patient Guide to Lung Cancer Testing.
CAP FAQs on Lung Cancer IHC Guidelines.

